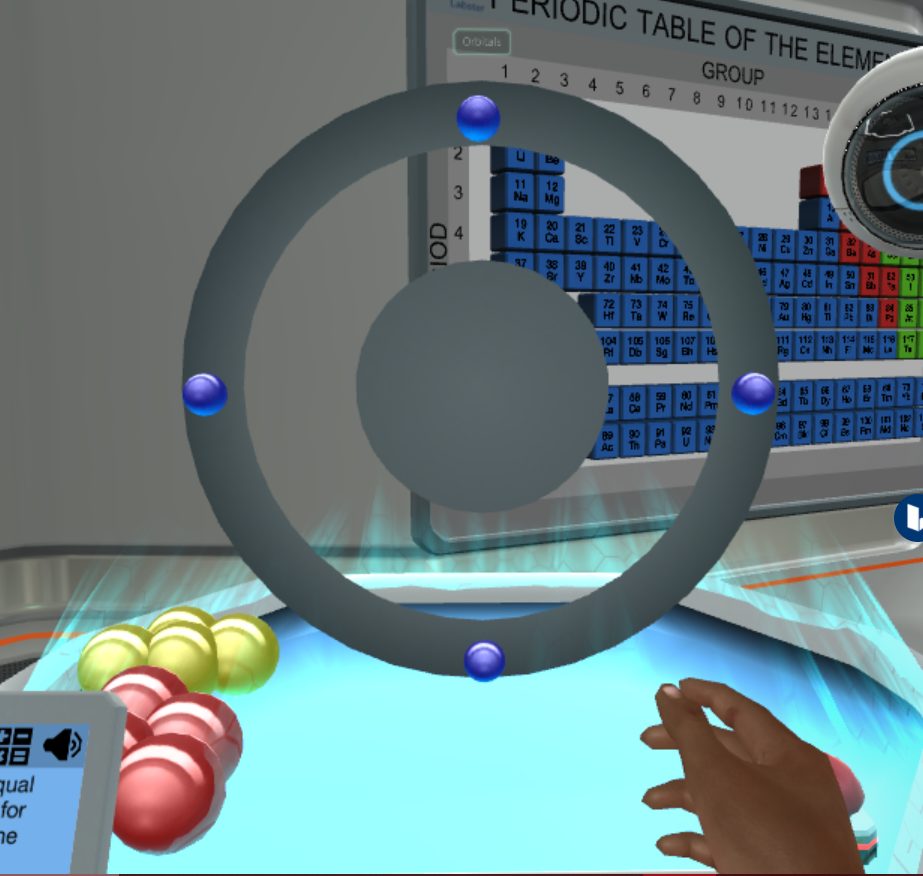
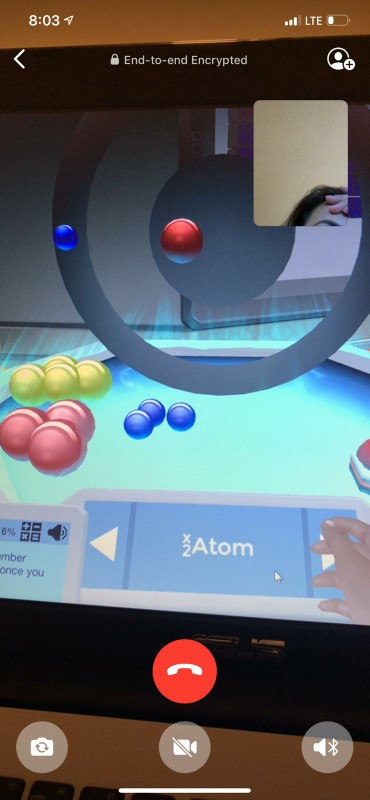
Create an Atomic Number Equal to 2
Atoms have no electric charge because they. Therefore its atomic number is 6.
Solved Score 10 110 Day 1 08 09 Progress 18 Create An Chegg Com
For example a lithium atom Z3 A7 amu contains three protons found from Z three electrons as the number of protons is equal to the number of electrons in an atom and four neutrons 7 3 4.
. The number of electrons and protons should be equal in a neutral atom. Atomic number the number of a chemical element in the periodic system whereby the elements are arranged in order of increasing number of protons in the nucleus. Isotopes all have the same number of protons but differ in the number of neutrons.
The sum of the number of the neutrons and electrons. Number of electrons in a neutral atom. Atomic number the number of a chemical element in the periodic system whereby the elements are arranged in order of increasing number of protons in the nucleus.
Click the submit button when you are finished. Determine the number o moles of ionsatomsparticle in the following. 140 arrow_forward An atom with atomic number 7 and a mass number of 14 is identical to an atom with atomic number of 6 and a mass number of 14.
According to the periodic table carbon contains six protons. See the answer See the answer See the answer done loading. The charge on the proton is equal but opposite to that of an electron.
Without using the simulation draw 2 atoms you have not yet made in the simulation. Create an isotope of the atom on the holotable. A Lithium atom with Atomic number 3 and Mass number 7.
2 Show answers Another question on Chemistry. Now make this isotope an anion. The atomic number of an element is equal to the number of protons in its nucleus.
Accordingly the number of protons which is always equal to the number of electrons in. The atomic number is equal to. Wiki User 2011-08-11 205809.
The atom having atomic number 2 is helium. A subatomic particle that has a negative charge is called a. Option 2 is correct ie.
Atomic number is equal to the number of protons. Create an atom with atomic number equal to 2. The sum of the number of protons and electrons.
You might be interested in. Click the red button when done. It is also the atomic mass number of the atom.
The number of the protons. The number of protons present in the nucleus is equal to the atomic number Z. Create an atom with an atomic number equal to two.
Click the red submit button when you are done. The presence of a positive charge on the nucleus is due to the protons in the nucleus. The atomic number is equal to number of electrons for nuclear atom.
Have an equal number of electrons and protons. 250 miles of k2s let me know how to do Answers. Create an atom with an atomic number equal to two I want shape.
There s two way to do it. This preview shows page 1 out of 1 page. Put the following onto the holotable.
Have an equal number of neutrons and protons. This problem has been solved. A neutral atom has an equal number of positive proton and negative electron charges.
Accordingly the number of protons which is always equal to the number of electrons in the neutral atom is also the atomic number. Chemistry 22062019 1200. The sum of the number of protons and neutrons.
Assuming the temperature remains constant what volume of ga. Create an atom with mass number equal to 3 and neutral charge. How many electrons are there in an atom with an atomic number of 45 and a mass number of 95 A.
It is also equal to the number of electrons in the atom. Atomic number is equal to the number of electrons. The atomic number is equal to number of protons and electrons.
Have an equal number of charged and non-charged particles. The atomic number can be used to uniquely identify ordinary chemical elementsIn an ordinary uncharged atom the. The atomic number or nuclear charge number symbol Z of a chemical element is the charge number of an atomic nucleusFor ordinary nuclei this is equal to the proton number n p or the number of protons found in the nucleus for every atom of that element.
Recall that the superscript refers to the mass number of an atom which is equal to the number of protons plus the number of neutrons present in an element. Have neutrons in the nuclei. Atomic number is also equal to number of protonsElements are arranged in order of atomic number in periodic tableThe atomic.
Atomic number number of protons. The sum of the number of protons neutrons and electrons. In a neutral atom the number of protons equals the number of electrons in shells that is the energy level around the nucleus.
Nata 24 1 year ago. Create an atom with an atomic number equal to two I want shape. Atomic number is proportional to atomic weight.
2 protons 1 neutron 2 electrons or 1 proton 2 neutrons 1 electron. Hence in order to create a neutral atom with a mass number of 3 the proton and neutron number must add up to 3 while the proton and electron number must be equal. 2 protons 1 neutron 2 electrons or 1 proton 2 neutrons 1 electron.
Create an atom with atomic number equal to 2. Atomic number Z is the number of protons in an atom. A container of gas has a volume of 35 L and a pressure of 08 atm.
Given an atomic number Z and mass number A you can find the number of protons neutrons and electrons in a neutral atom. The atomic number of an element is the number of this element in the Periodic Table of Mendeleev. Asked Jul 14 2019 in Chemistry by Kimberly.
The atomic number of an element is 12 then its atom contains 12 protons and 12 electrons. Because each electron has a -1 charge and each proton a 1 charge the number of each must be the same for the net charge to equal zero. Isotopes are atoms with the same atomic number but distinct neutron numbers and hence distinct mass numbers.
Solve any question of Structure of Atom with-. Theres two way to do it.

4 16 Atomic Number Chemistry Libretexts

Solved Create An Atom With An Atomic Number Equal To Two Chegg Com

3 4 Atomic Mass And Atomic Number Chemistry Libretexts Periodic Table Mass Number Atomic Number
Comments
Post a Comment